- Primary care
- General practice
- Pediatric inhalation chamber
- OptimHal Laboratoire

- Products
- Catalogs
- News & Trends
- Exhibitions
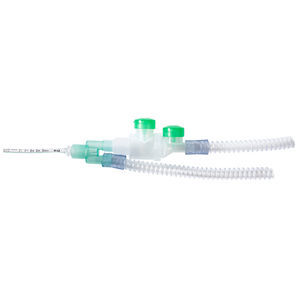
Patient breathing circuit inhalation chamber CombiHaler™pediatric
Add to favorites
Compare this product
Characteristics
- Applications
- pediatric, patient breathing circuit
Description
The CombiHaler® is 100% designed and manufactured in France. CombiHaler® spacer for mechanical ventilation and critical care (ICU) enables the use of both a vibrating mesh nebuliser such as Aerogen®, and a pMDI at the same time.
Specifications of the spacer CombiHaler®
CombiHaler® is a device aimed to improve respiratory drug deposition for ventilated patients in critical care (ICU). It was designed to enable the use of a pMDI and an Aerogen® together.
Description of the device and its intended use
CombiHaler® is a spacer designed to be integrated on a breathing device circuit in invasive or non-invasive ventilation. It enables the connection of a pMDI and/or an Aerogen® Pro or an Aerogen® Solo without intervention on circuit.
CombiHaler® is intended to administer drugs by both nebulizer and pressurized metered dose inhaler (pMDI). It limits in particular the impaction deposit or the substance in the spacer.
The CombiHaler® spacer is single use only and is meant to be used in hospital, in intensive care services or for home care.
Who is it for ?
It is intended for patients suffering from Chronic Obstructive Pulmonary Disease (COPD), asthma or chronic obstructive respiratory insufficiency in pulmonology, critical care or operating suite.
How does the CombiHaler® spacer work ?
The spacer is meant to be crossed by gas flows in a circuit of a device for mechanical ventilation.
CombiHaler® has four openings leading to its inside volume. The CombiHaler® spacer has two openings meant to be connected to the respirator circuit: one openings is meant for the way in of the gas flow and the other one is meant for the way out of the gas flow.
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.