- Secondary care
- Orthopedic surgery
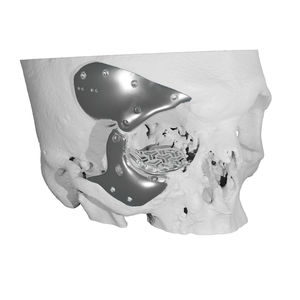
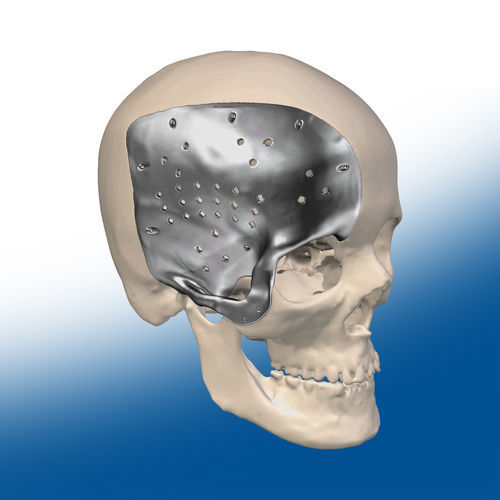
- Custom-made cranial implant
- Ortho Baltic Implants

- Company
- Products
- Catalogs
- News & Trends
- Exhibitions
Custom-made cranial implant

Add to favorites
Compare this product
Characteristics
- Type of implant
- custom-made
Description
Patient-specific cranial implants are plate-type medical devices that differ by their size and configurations (depending on the size and location of the cranial defect) and that are used in cranioplasty to correct cranial defects. The main goals of cranioplasty are to prevent injuries and infection of the unprotected brain, to correct cranial deformations and restore the aesthetic of the skull.
Nowadays in cranioplasty, the following ways are mainly used to fill cranial defects: bone grafts, mesh, standard cranial implants, patient-specific cranial implants, and quick-hardening polymeric substances (PMMA). The latter way is relatively cheap; however, when using PMMA, the implant is formed directly on the soft tissues that cover the cerebral cortex, even though this quick-hardening material emits a temperature of 72°–86°C (for comparison it can be noted that changes in the soft tissues start to occur from 42°C). Moreover, using PMMA, cranial bone aesthetic results are usually unsatisfying.
Usually, there are two types of bone grafts used in cranioplasty: autografts (or autogenous bone grafts) and allografts. The difference between them is the fact that the autograft is the bone flap that was obtained from the same patient who received it, whereas allograft is a graft from a person other than the one receiving it. The use of autografts is only possible during primary (planned) cranioplasty and they cannot be used when treating high energy head trauma defect when the cranial bone is too severely damaged.
VIDEO
Catalogs
No catalogs are available for this product.
See all of Ortho Baltic Implants‘s catalogsOther Ortho Baltic Implants products
Patient-specific Medical Devices
Related Searches
- Bone plate
- Compression plate
- Metallic compression plate
- Acetabular prosthesis
- Revision acetabular prosthesis
- Cemented acetabular prosthesis
- Cranial implant
- Custom-made cranial implant
- Periorbital implant
- Resection guide
- Custom-made periorbital implant
- Custom-made temporomandibular implant
- Hip prosthesis resection guide
- Temporomandibular implant
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.