- Laboratory
- Laboratory medicine
- Drug detection test kit
- R-Biopharm AG
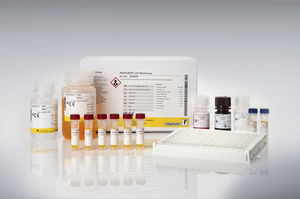
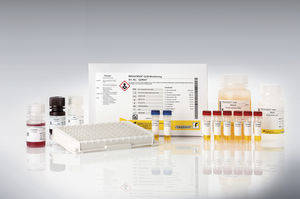
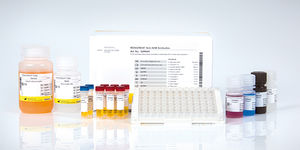
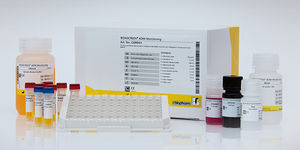
Drug detection test kit RIDASCREEN®serumplasmaELISA
Add to favorites
Compare this product
Characteristics
- Application field
- drug detection
- Sample type
- serum, plasma
- Analysis mode
- ELISA, automated
- Result display time
100 min
Description
The RIDASCREEN® VDZ Monitoring is an enzyme linked immunosorbent assay intended for the quantitative determination of vedolizumab (VDZ, Entyvio®, anti- integrin α4β7) in human serum and plasma.
Product information
Vedolizumab (VDZ, Entyvio®) is approved for the treatment of patients with moderate to severe Crohn’s disease and ulcerative colitis in whom conventional therapy or anti-TNFα therapies were ineffective or not tolerated. It is a gut-specific, humanized monoclonal antibody targeting the α4β7-integrin protein, which is involved in the migration of lymphocytes to the gut. Similar as to anti-TNFα drugs, measurement of vedolizumab drug concentrations supports a personalized dosing strategy in patients treated with vedolizumab.
The RIDASCREEN® VDZ Monitoring and our other TDM assays are vital to personalize treatment of patients with Crohn’s disease and ulcerative colitis and better achieve therapeutic targets.
Key features:
CE-marked version of the ELISA tests of KU Leuven
Highly specific antibodies
Ready to use reagents
Validated for use on automated ELISA systems, e.g. the Dynex DSX®
Catalogs
Related Searches
- Assay kit
- Solution reagent kit
- Blood assay kit
- Serum assay kit
- Immunoassay assay kit
- Plasma assay kit
- Infectious disease detection kit
- Diagnostic reagent kit
- Rapid lateral flow test
- Immunoassay rapid diagnostic test
- Molecular test kit
- Respiratory infection test kit
- Clinical assay kit
- Infectious disease rapid diagnostic test
- Cassette assay kit
- Lateral flow test kit
- COVID-19 detection kit
- ELISA assay kit
- Real-time PCR test kit
- Quality control reagent kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.