Gastrointestinal infection test kit RIDA®UNITYSalmonellaYersinia enterocoliticaclinical
Add to favorites
Compare this product
Characteristics
- Applications
- for gastrointestinal infections
- Micro-organism
- Salmonella, Yersinia enterocolitica
- Sample type
- clinical, laboratory, stool
- Analysis mode
- for real-time PCR
Description
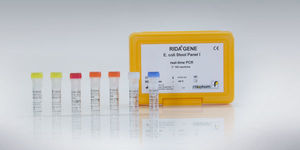
For in-vitro diagnostics. RIDA®UNITY Bacterial Stool Panel is a multiplex real-time PCR for direct qualitative detection and differentiation of Campylobacter spp. (C. coli, C. lari, C. jejuni), Salmonella spp. and Yersinia enterocolitica DNA from untreated human stool samples from individuals with signs and symptoms of acute gastroenteritis. It is only approved for processing on the RIDA®UNITY platform.
The RIDA®UNITY Bacterial Stool Panel Test is intended to aid in the differential diagnosis of bacterial infections (Campylobacter spp. (C. coli, C. lari, C. jejuni), Salmonella spp. and Yersinia enterocolitica) in patients with symptoms of gastroenteritis in conjunction with other clinical and laboratory findings.
General information:
Diarrheal illnesses are a significant health problem, causing approximately 1.7 billion cases per year in children worldwide. According to the World Health Organization (WHO), at approximately 525,000 deaths per year, they are the second leading cause of death in children under the age of 5, especially in developing countries. Campylobacter spp., Salmonella spp., and Yersinia enterocolitica infections are common causes of cases of bacterial diarrhea.
Worldwide, Campylobacter species are one of the four most common causes of bacterial diarrhea. In the USA, these pathogens cause approximately 1.5 million cases (campylobacteriosis) per year.
Catalogs
No catalogs are available for this product.
See all of R-Biopharm AG‘s catalogsRelated Searches
- Assay kit
- Solution reagent kit
- Blood assay kit
- Serum assay kit
- Immunoassay assay kit
- Plasma assay kit
- Infectious disease detection kit
- Rapid lateral flow test
- Diagnostic reagent kit
- Immunoassay rapid diagnostic test
- Molecular test kit
- Respiratory infection test kit
- Infectious disease rapid diagnostic test
- Clinical assay kit
- Cassette assay kit
- Lateral flow test kit
- ELISA assay kit
- COVID-19 detection kit
- Real-time PCR test kit
- Quality control reagent kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.