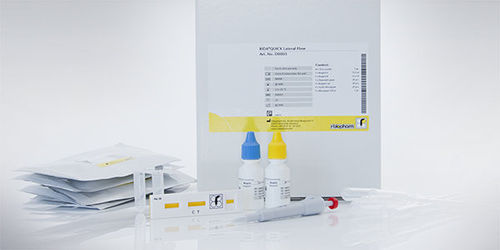
Hospital-acquired infection test kit RIDA®QUICKClostridium difficilestoolimmunochromatographic
Add to favorites
Compare this product
Characteristics
- Applications
- for hospital-acquired infections
- Micro-organism
- Clostridium difficile
- Sample type
- stool
- Analysis mode
- immunochromatographic
Description
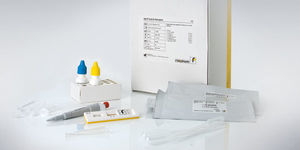
For in vitro diagnostic use. RIDA®QUICK Clostridium difficile Toxin A/B is an immunochromatographic rapid assay for the qualitative determination of the toxins A and B of Clostridium difficile in stool samples and in culture supernatants.
General information:
The increase in frequency and increasing severity of Clostridioides (Clostridium) difficle infection (CDI) leads to rising healthcare expenses, and increased morbidity and mortality. The implementation of multistage testing algorithms has improved overall diagnostic accuracy being of crucial importance for therapy decisions.
RIDA®QUICK Clostridium difficile Toxin A/B supports CDI detection:
Direct determination of free C. difficile toxin A and toxin B in the patient specimen
Elaborated antibody mixture for high specificity
Obtains reliable results in combination with RIDA®QUICK Clostridium difficile GDH
Clear result readout with colloidal-gold particles
Catalogs
No catalogs are available for this product.
See all of R-Biopharm AG‘s catalogsRelated Searches
- Assay kit
- Solution reagent kit
- Blood assay kit
- Serum assay kit
- Immunoassay assay kit
- Plasma assay kit
- Infectious disease detection kit
- Diagnostic reagent kit
- Rapid lateral flow test
- Immunoassay rapid diagnostic test
- Molecular test kit
- Respiratory infection test kit
- Clinical assay kit
- Infectious disease rapid diagnostic test
- Cassette assay kit
- Lateral flow test kit
- COVID-19 assay kit
- ELISA assay kit
- Real-time PCR test kit
- Quality control reagent kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.