- Laboratory
- Laboratory medicine
- Pneumonia test kit
- R-Biopharm AG
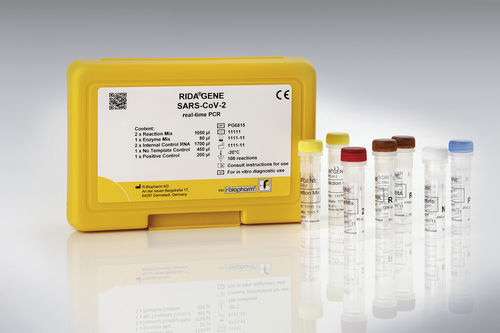
COVID-19 test kit RIDA®GENEpneumoniaSARS-COV-2coronavirus
Add to favorites
Compare this product
Characteristics
- Applications
- COVID-19, pneumonia
- Micro-organism
- SARS-COV-2, coronavirus
- Sample type
- clinical, laboratory, nasal, throat
- Analysis mode
- for real-time PCR
Description
For in vitro diagnostic use. The RIDA®GENE SARS-CoV-2 test, which will be performed on the Roche LightCycler® 480II, is a multiplex real-time RT-PCR for the direct qualitative detection of coronavirus (SARS-CoV-2) RNA from human nasal/throat swabs of people with symptoms of a respiratory infection.
The RIDA®GENE SARS-CoV-2 test is designed to support the differential diagnosis of SARS-CoV-2 infections in patients with symptoms of respiratory infection in conjunction with other clinical and laboratory findings.
Negative results do not rule out infection with SARS-CoV-2 and should not be used as the sole basis for diagnosis.
The product is intended for use by professional users in hospital laboratories, reference laboratories, private laboratories or state laboratories.
The RIDA®GENE SARS-CoV-2 multiplex real-time RT-PCR test is compatible for use with the following real-time PCR devices: LightCycler® 480 II, RIDA®CYCLER, Mx3005P, ABI 7500 Fast Dx, CFX96™ Dx, Rotor-Gene Q.
General information:
At the end of December in the Chinese metropolis of Wuhan, numerous cases of pneumonia of unknown cause occurred. At the beginning of January, Chinese authorities identified a new type of corona virus (SARS-CoV-2) as the cause. The disease caused by SARS-CoV-2 is officially named COVID-19 („Corona Virus disease 2019“) and is transmissible from person to person.
Worldwide, 3,925,815 cases have been reported (as of May 10, 2020). The initial cases in Germany were confirmed at the end of January 2020. In Germany 169,575 cases have been reported (as of May 11, 2020). The WHO declared an international health emergency on January 31, 2020.
Catalogs
Related Searches
- Assay kit
- Solution reagent kit
- Blood assay kit
- Serum assay kit
- Immunoassay assay kit
- Plasma assay kit
- Infectious disease detection kit
- Diagnostic reagent kit
- Rapid lateral flow test
- Immunoassay rapid diagnostic test
- Molecular test kit
- Respiratory infection test kit
- Clinical assay kit
- Infectious disease rapid diagnostic test
- Cassette assay kit
- Lateral flow test kit
- COVID-19 detection kit
- ELISA assay kit
- Real-time PCR test kit
- Quality control reagent kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.