- Laboratory
- Laboratory medicine
- Flu test kit
- R-Biopharm AG
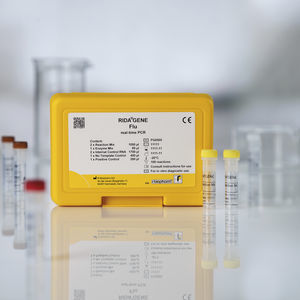
Flu test kit RIDA®GENErespiratory syncytial virusRSVclinical
Add to favorites
Compare this product
Characteristics
- Applications
- flu
- Micro-organism
- respiratory syncytial virus, RSV
- Sample type
- clinical, laboratory, nasal, throat
- Analysis mode
- for real-time PCR
Description
For in vitro diagnostic use. The RIDA®GENE Flu & RSV test, performed on the Roche LightCycler® 480II, is a multiplex real-time RT-PCR for the direct qualitative detection and differentiation of influenza virus RNA (influenza A and influenza B) and respiratory syncytial virus RNA (RSV A and B) in human nasal/throat swabs and BAL from persons with signs and symptoms of acute respiratory infection.
The RIDA®GENE Flu & RSV test is intended to support the differential diagnosis of infections caused by influenza viruses (influenza A and influenza B) and respiratory syncytial viruses (RSV A and B) in patients with symptoms of a respiratory infection in connection with other clinical and laboratory findings.
Negative results do not rule out infection with influenza viruses (influenza A, influenza B) or respiratory syncytial viruses (RSV A and B) and should not be used as the sole basis for diagnosis. The product is intended for use by professionals working in hospital laboratories, reference laboratories, private laboratories, or public laboratories.
General information:
Also called the flu, influenza is one of the most significant infectious respiratory diseases, and it is caused by influenza viruses.
Worldwide three to five million people contract influenza every year, and approximately 290,000 to 650,000 people die from the illness. The annual influenza epidemics can have major impacts on the health care system and the economy.
Catalogs
No catalogs are available for this product.
See all of R-Biopharm AG‘s catalogsRelated Searches
- Assay kit
- Solution reagent kit
- Blood assay kit
- Serum assay kit
- Immunoassay assay kit
- Plasma assay kit
- Infectious disease detection kit
- Diagnostic reagent kit
- Rapid lateral flow test
- Immunoassay rapid diagnostic test
- Molecular test kit
- Respiratory infection test kit
- Clinical assay kit
- Infectious disease rapid diagnostic test
- Cassette assay kit
- Lateral flow test kit
- COVID-19 detection kit
- ELISA assay kit
- Real-time PCR test kit
- Quality control reagent kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.