
- Company
- Products
- Catalogs
- News & Trends
- Exhibitions
Sterility test closed canister Sterisart® NF
Add to favorites
Compare this product
Characteristics
- Applications
- sterility test
Description
The International Pharmacopeia requires that each batch of products sold as sterile articles must be tested and certified for sterility prior to their sale and distribution. Therefore, sterility testing is part of the validation process as well as of routine release testing. For these dedicated tests, trained personnel, a regulated environment and high-quality materials that provide adequate and reliable sterility test data are absolutely essential. The comprehensive Sterisart® solution for sterility testing, which consists of hardware and single-use components as well as training and other related services, exactly meets all these requirements.
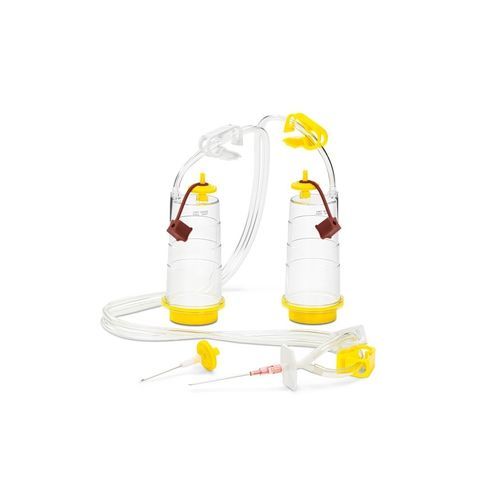
Sterisart® NF consumables are single-use units that constitute closed systems for performing sterility tests on open or closed containers.
The Sterisart® NF System is a completely closed system for the sterility testing of pharmaceutical products. It is based on the membrane filter method, however it eliminates the procedure of manipulating the filters. By this means the main risk of a secondary contamination and false positive results is eliminated.
Overview
Unique Septum Version for aspetic sampling
High retention of microbes
Low adsorption
High mechanical stability
Sharp Metal needle for easy penetration
Pre-installed color-coded tube clamps
Easy-to-read graduated marks
User-friendly (several practical adapters available)
Product-/lot number identification
Huge variety of adapters and more than 20 different unit types
Gas-impermeable packaging for protection against sterilants
Catalogs
Sterisart® NF
4 Pages
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.