- Company
- Products
- Catalogs
- News & Trends
- Exhibitions
Electric pump Sterisart®
Add to favorites
Compare this product
Characteristics
- Operation
- electric
Description
The International Pharmacopeia requires that each batch of products sold as sterile articles must be tested and certified for sterility prior to their sale and distribution. Therefore, sterility testing is part of the validation process as well as of routine release testing. For these dedicated tests, trained personnel, a regulated environment and high-quality materials that provide adequate and reliable sterility test data are absolutely essential. The comprehensive Sterisart® solution for sterility testing, which consists of hardware and single-use components as well as training and other related services, exactly meets all these requirements.
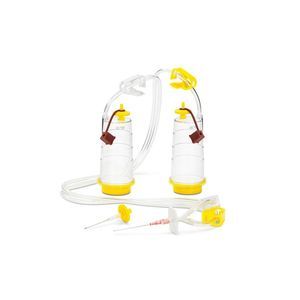
The Sterisart® Universal Pump enables contamination-free, safe transfer of samples and media into the Sterisart® NF system.
Sterisart® universal pump for sample and media transfer into the Sterisart® NF systems for sterility tests. The peristaltic pump can be used in clean rooms, integrated into clean benches, or installed countersunk in the working surface of isolators. Sterisart® universal pump is available as an upgraded version with display and user software. The sterility test system features a compact design with a space-saving footprint - a great benefit for most clean room benchtops and isolators.
Overview
The Sterisart® Universal Pump is available in two versions: as basic version 16419 and as an upgraded version 16420 with display and user software.The pump can be used in clean rooms, integrated into clean benches, or installed countersunk in the working surface of isolators. Its low, compact design has a space-saving footprint – a great benefit for most clean room benchtops and isolators.
Catalogs
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.