- Secondary care
- Cardiology
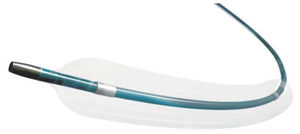
- Coronary stent
- SCITECH Medical
Coronary stent Inspironpolymerdrug eluting
Add to favorites
Compare this product
Characteristics
- Type
- coronary arteries
- Material
- polymer
- Options
- drug eluting
Description
INSPIRON® is a 3rd generation Drug Eluting Stent designed to create a fast and homogeneous endothelialization. Its platform is the CRONUS NE® Stent, a stent which design, material (CoCr), thin structures (75 μm) and delivery system gives it good navigability, flexibility, good crossover profile, moderate radiopacity and high balloon rupture pressure. Its abluminal coating is composed of a mixture of PLA and PLGA polymers and a low Sirolimus dosage resulting in a moderate drug elution profile (60% in 10 days and 100% in 45%), complete coating degradation between 6 and 9 months and in a fast and homogeneous endothelialization.
Inspiron DES is a Sirolimus eluting stent with abluminal coating and biodegradable polymer in a Cobalt Chromium platform.
Inspiron is the first Drug Eluting made in Brazil under international standards (EN ISO 25539-2 & US FDA guidance for industry) resulting in a safe product.
Indication:
Percutaneous Transluminal Coronary Angioplasty (PTCA)
Main characteristics:
- Abluminal Coating
- Biodegradable polymer
- Sirolimus Elution
- Moderate drug release profile (60% in 10 days and the remainder in 45 days)
- In 6 to 9 months the polymer is completely degraded
- Cobalt Chromium Platform
- High Flexibility
- Great side branch access
- Thinner Links (80 μm)
Catalogs
No catalogs are available for this product.
See all of SCITECH Medical‘s catalogs*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.