- Company
- Products
- Catalogs
- News & Trends
- Exhibitions
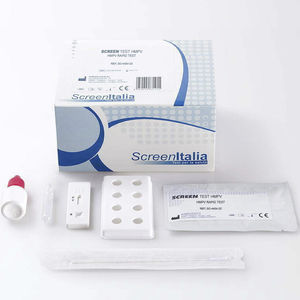
Rapid infectious disease test SC-2368-25for antibodiesmonkeypoxserum
Add to favorites
Compare this product
Characteristics
- Applications
- for infectious diseases
- Tested parameter
- for antibodies
- Micro-organism
- monkeypox
- Sample type
- serum, plasma, whole blood
- Analysis mode
- lateral flow
- Format
- cassette
- Other characteristics
- self-test, with digital reader
- Result display time
Min.: 10 min
10 min
Max.: 20 min
- Specificity
Min.: 95.88 %
95 %, 98.57 %
Max.: 99.7 %
- Sensitivity
Min.: 55.5 %
90 %, 98.6 %
Max.: 99.8 %
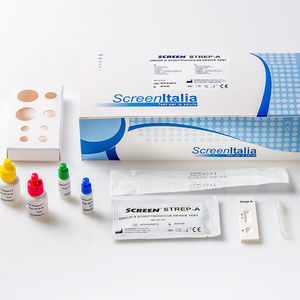
Description
SCREEN TEST VAIOLO DELLE SCIMMIE
A rapid test for the qualitative detection of antibodies to Monkeypox Virus in human whole blood, serum or plasma. For professional in vitro diagnostic use only. The Monkeypox Virus Antibody Rapid Test is a lateral flow chromatographic immunoassay for the qualitative detection of antibodies to Monkeypox Virus in whole blood, serum or plasma to aid in the diagnosis of Monkeypox Virus infection. Monkeypox is a zoonotic orthopoxvirus that incidentally causes disease in humans similar to smallpox, although with notably lower mortality. This virus is clinically relevant because it is endemic to western and central Africa, with outbreaks in the Western Hemisphere related to the exotic pet trade and international travel. Transmission can occur through contact with bodily fluids, skin lesions, or respiratory droplets of infected animals directly or indirectly via contaminated fomites. Although humanto-human transmission has previously been limited, mathematical modeling in the context of decreasing herd immunity to orthopoxviruses reflects an increasing threat of disease spread between humans. The Centers for Disease Control and Prevention (CDC) recommends isolation in a negative pressure room and standard, contact, and droplet precautions in the healthcare setting with escalation to airborne precautions if possible. Following viral entry from any route (oropharynx, nasopharynx, or intradermal), the monkeypox virus replicates at the inoculation site then spreads to local lymph nodes. Next, an initial viremia leads to viral spread and seeding of other organs.
Catalogs
SCREEN CATALOGUES EN
182 Pages
Related Searches
- Assay kit
- Blood assay kit
- Immunoassay assay kit
- Blood rapid diagnostic test
- Rapid lateral flow test
- Immunoassay rapid diagnostic test
- Cassette rapid diagnostic test
- Rapid virus test
- Respiratory infection test kit
- Serum rapid diagnostic test
- Plasma rapid diagnostic test
- Infectious disease rapid diagnostic test
- Whole blood rapid diagnostic test
- Cassette assay kit
- Lateral flow test kit
- COVID-19 detection kit
- Rapid respiratory infection test
- Urine rapid diagnostic test
- Antigen assay kit
- COVID-19 rapid diagnostic test
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.