- Laboratory
- Laboratory medicine
- Flu test kit
- Shenzhen kangshengbao Biotechnology Co., Ltd.

- Products
- Catalogs
- News & Trends
- Exhibitions
Infectious disease test kit fluinfluenza Alaboratory
Add to favorites
Compare this product
Characteristics
- Applications
- for infectious diseases, flu
- Micro-organism
- influenza A
- Sample type
- laboratory, nasal, cell, throat
- Analysis mode
- immunochromatographic
Description
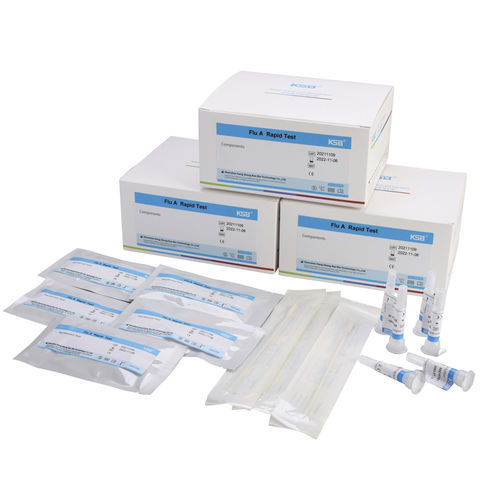
10 tests/ kit
registration number:CE
Test Device (10 tests)
Lysis Solution (12ml)
Extraction Tube (10)
Sterile Nasal Swab (10)
Intended use
Flu A qualitative rapid test Kit is an in vitro immunochromatographic assay for the qualitative detection of Influenza type A viral antigen from nasal swab, throat swab or nasal wash/aspirate specimens. It is intended to aid in the rapid differential diagnosis of influenza type A viral infections. Negative test results should be confirmed by cell culture because negative results do not preclude influenza virus infection and should not be used as the sole basis for treatment or other management decisions. The test is intended for professional and laboratory use.
Other Shenzhen kangshengbao Biotechnology Co., Ltd. products
Infectious Markers
Related Searches
- Assay kit
- Blood assay kit
- Serum assay kit
- Immunoassay assay kit
- Plasma assay kit
- Infectious disease detection kit
- Blood rapid diagnostic test
- Rapid lateral flow test
- Immunoassay rapid diagnostic test
- Rapid virus test
- Whole blood detection kit
- Respiratory infection test kit
- Serum rapid diagnostic test
- Plasma rapid diagnostic test
- Clinical assay kit
- Infectious disease rapid diagnostic test
- Whole blood rapid diagnostic test
- Lateral flow test kit
- Rapid respiratory infection test
- Antigen assay kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.