- Laboratory
- Laboratory medicine
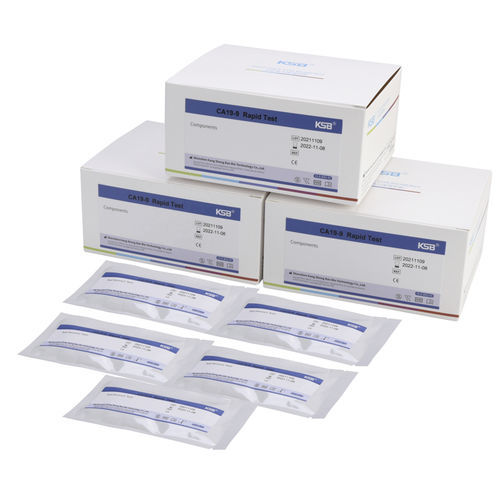
- Pancreatic cancer rapid test
- Shenzhen kangshengbao Biotechnology Co., Ltd.

- Products
- Catalogs
- News & Trends
- Exhibitions
Rapid CA 19-9 test for pancreatic canceroncologyserum
Add to favorites
Compare this product
Characteristics
- Applications
- for pancreatic cancer
- Application field
- oncology
- Tested parameter
- CA 19-9
- Sample type
- serum, plasma, clinical, laboratory
- Analysis mode
- chromatographic immunoassay
Description
20 tests/ kit
registration number:CE
product compasition:
Test Device: 20 Tests
1 instruction for use
SD card ( with specific standard curve file)
Intended use
CA 19-9 quantitative rapid test Kit is a chromatographic immunoassay for quantitative measurement of the CA 19-9 tumor associated antigen in human serum or plasma, is indicated for the serial measurement of CA 19-9 to aid in the management of patients diagnosed with cancers of the exocrine pancreas. The test is useful as an aid in: Monitoring of disease status in those patients having confirmed pancreatic cancer who have levels of serum or plasma CA19-9 above the cutoff, at the time of diagnosis.
CA 19-9 values must be interpreted in conjunction with all other available clinical and laboratory data before a medical decision is determined.
Other Shenzhen kangshengbao Biotechnology Co., Ltd. products
Tumour Markers
Related Searches
- Assay kit
- Blood assay kit
- Serum assay kit
- Immunoassay assay kit
- Plasma assay kit
- Infectious disease detection kit
- Blood rapid diagnostic test
- Rapid lateral flow test
- Immunoassay rapid diagnostic test
- Respiratory infection test kit
- Rapid virus test
- Whole blood detection kit
- Serum rapid diagnostic test
- Plasma rapid diagnostic test
- Clinical assay kit
- Infectious disease rapid diagnostic test
- Whole blood rapid diagnostic test
- Lateral flow test kit
- Rapid respiratory infection test
- Antigen assay kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.