- Laboratory
- Laboratory medicine
- Rapid infectious disease test
- Shenzhen unimed-global technology Co.,LTD

- Products
- Catalogs
- News & Trends
- Exhibitions
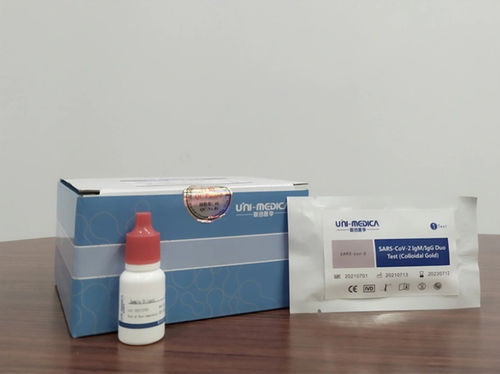
Rapid infectious disease test 300215IgGIgMSARS-COV-2
Add to favorites
Compare this product
Characteristics
- Applications
- for infectious diseases
- Tested parameter
- IgG, IgM
- Micro-organism
- SARS-COV-2, coronavirus
- Sample type
- serum, plasma, whole blood
- Result display time
Max.: 15 min
Min.: 5 min
- Sample volume
0.02 ml
(0.00068 US fl oz)
Description
This rapid antibody test kit is used for in vitro qualitative detection of new coronavirus (SARS-CoV-2) IgM/IgG antibodies in human serum/plasma/whole blood samples.
【Instructions】
1. Please read the instruction manual completely before testing, and return the test card and sample to room temperature and number before use.
2. Take out the test card from the packaging bag, place the test card on a clean horizontal table, place it horizontally and make a mark.
3. Take 10 μL of serum/plasma or 20 μL of whole blood and add it vertically to the sample hole of the test card, and then immediately add 2 drops of sample buffer to the sample hole of the test card.
[Explanation of test results]
1. Positive result (+)
a) If the quality control line (C line) and the detection line M line and G line are all colored, it means that the new coronavirus IgM antibody and IgG antibody are detected, and the result is judged to be IgM antibody positive and IgG antibody positive (as shown below)
b) If both the quality control line (C line) and the detection line M line are colored, it means that the new coronavirus IgM antibody has been detected, and the result is judged to be IgM antibody positive (as shown below)
c) If both the quality control line (C line) and the test line G line are colored, it means that the new coronavirus IgG antibody is detected, and the result is judged to be IgG antibody positive (as shown below)
2. Negative result (-)
If only the quality control line (C line) appears, and the detection line G line and M line are not colored, it means that no new coronavirus antibody has been detected, and the result is judged as negative (as shown below)
Other Shenzhen unimed-global technology Co.,LTD products
Colloidal Gold Platform
Related Searches
- Assay kit
- Solution reagent kit
- Blood assay kit
- Molecular biology reagent kit
- Serum assay kit
- Immunoassay assay kit
- Plasma assay kit
- Infectious disease detection kit
- Blood rapid diagnostic test
- Protein reagent kit
- Diagnostic reagent kit
- Rapid lateral flow test
- Immunoassay rapid diagnostic test
- Molecular test kit
- Cassette rapid diagnostic test
- Rapid virus test
- Respiratory infection test kit
- Whole blood detection kit
- Serum rapid diagnostic test
- Plasma rapid diagnostic test
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.