- Laboratory
- Laboratory medicine
- Solution reagent
- Shenzhen unimed-global technology Co.,LTD

- Products
- Catalogs
- News & Trends
- Exhibitions
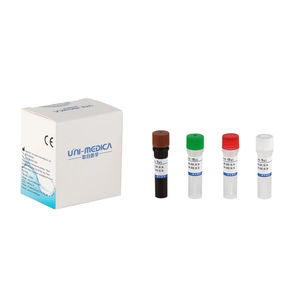
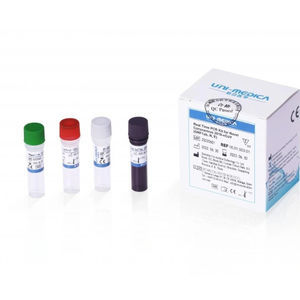
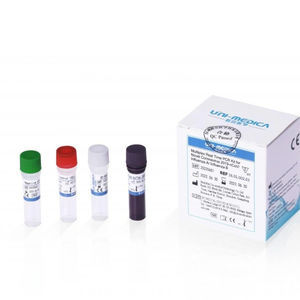
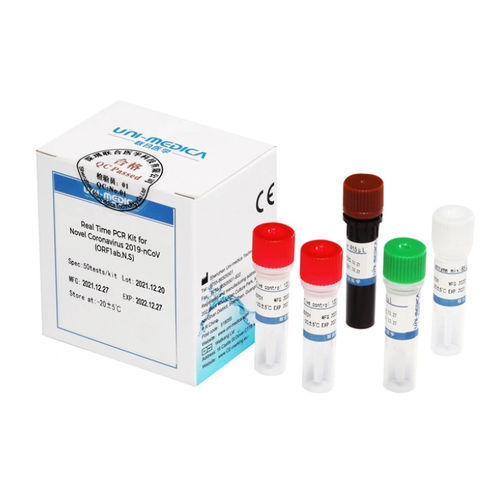
Solution reagent kit 382200for PCRcoronavirusSARS-Cov-2 omicron
Add to favorites
Compare this product
Characteristics
- Type
- solution
- Applications
- for PCR
- Micro-organism
- coronavirus, SARS-Cov-2 omicron, SARS-COV-2
- Storage temperature
-25 °C, -20 °C, -15 °C
(-13 °F, -4 °F, 5 °F)
Description
The Omicron Test reagent kit is used for in vitroqualitative detecting theORF1ab/N/S genes of novel coronavirus 2019-nCoV in respiratory specimens including oropharyngeal swabs, nasopharyngeal swab, sputum and bronchoalveolar lavage fluid, and performing mutation typing on HV69-70 del simultaneously. As reported in GISAID database, more than 95% Omicron variant sequences reported include a deletion in theHV69-70, which can cause an S gene target failure (SGTF) in PCR assays. SGTF can be used as a proxy marker to screen for Omicron.
Primer sets and FAM labeled probe are designed for specific detection of ORFlab gene of 2019-nCoV, VIC labeled probe for N gene of 2019-nCoV, ROX labeled probe for S gene HV69-70 del mutation of 2019-nCoV. Human RNase P gene extracted concurrently with the test sample provides an internal control to validate nucleic extraction procedure and reagent integrity. Probe targeting human RNase P gene is labeled withCY5.
This product is only used for primary shunting of patients with COVID-19.
Storage and Expiry Date
1.This New coronavirus test reagent kit expires in 12 months when stored at -20±5℃ and in prevention of light.
2.Avoid repeated freeze-thaw cycles (5 times maximum).
3.Manufacture date and expiration date are printed on the label.
Applicable instrument
real-time pcr system :
Real-Time PCR System: Molarray MA-6000, ABI 7500, ViiATM7, QuantStudio 5, QuantStudio 6/7 pro, QuantStudio 6/7 flex, Agilent Mx3000P/3005P, Rotor-GeneTM6000/Q, Bio-Rad CFX96 TouchTM/iQTM5,Hongshi SLAN-96S/96P,AGS8830, AGS4800, etc.
Our Advantage
1. High sensitivity: 100-200 copies/ml.
2. Short amplification time: 50 mins
3. Quick delivery time: 1-3 days.
Other Shenzhen unimed-global technology Co.,LTD products
PCR Test Reagent Kit Related With COVID-19
Related Searches
- Assay kit
- Solution reagent kit
- Blood assay kit
- Molecular biology reagent kit
- Serum assay kit
- Immunoassay assay kit
- Plasma assay kit
- Infectious disease detection kit
- Blood rapid diagnostic test
- Protein reagent kit
- Diagnostic reagent kit
- Rapid lateral flow test
- Immunoassay rapid diagnostic test
- Molecular test kit
- Cassette rapid diagnostic test
- Rapid virus test
- Respiratory infection test kit
- Whole blood detection kit
- Serum rapid diagnostic test
- Plasma rapid diagnostic test
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.