- Laboratory
- Laboratory medicine
- Immunity test kit
- Shenzhen unimed-global technology Co.,LTD

- Products
- Catalogs
- News & Trends
- Exhibitions
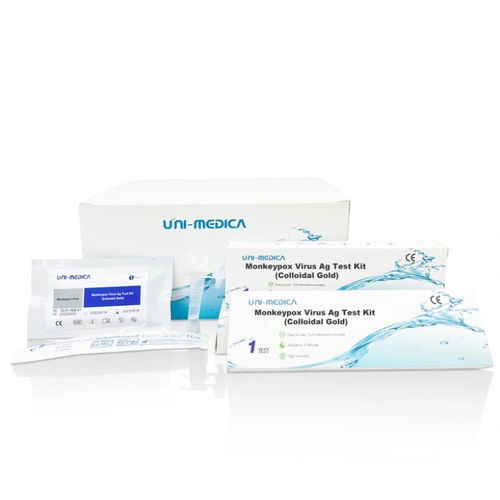
Immunity test kit for antigensmonkeypoxclinical
Add to favorites
Compare this product
Characteristics
- Application field
- immunity
- Tested parameter
- for antigens
- Micro-organism
- monkeypox
- Sample type
- clinical, laboratory, saliva
- Format
- cassette
- Result display time
10 min
- Sample volume
0.3 ml
(0.01014 US fl oz)- Sensitivity
99 %
Description
The Monkeypox Virus Ag Test Kit is a gold immuno-chromatographic assay (GICA) that is intended for the qualitative detection of the nucleocapsid protein Antigen from Monkeypox Virus in oropharyngeal swaband saliva specimens directly from individuals who are suspected of Monkeypox Virus by their healthcare provider, with or without signs and symptoms of Monkeypox Virus. Results are for the identification of Monkeypox Virus nucleocapsid protein antigen. Antigen is generally detectable in upper respiratory specimens during the acute phase of infection. Positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected
may not be the definite cause of disease. Negative results should be treated as presumptive and confirmed with a molecular assay, if necessary for patient management. Negative results do not rule out Monkeypox Virus and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions.
Negative results should be considered in the context of a patient’s recent exposures, history and the presence of clinical signs and symptoms consistent with Monkeypox Virus.
The Monkeypox Virus Ag Test Kit is intended for use by trained clinical laboratory personnel and individuals trained in point of care settings.
Storage and Expiry Date
2. The Test Cassette should be used within 2 hour once it has been removed from the foil pouch.
Other Shenzhen unimed-global technology Co.,LTD products
Colloidal Gold Platform
Related Searches
- Assay kit
- Solution reagent kit
- Blood assay kit
- Molecular biology reagent kit
- Serum assay kit
- Immunoassay assay kit
- Plasma assay kit
- Infectious disease detection kit
- Blood rapid diagnostic test
- Protein reagent kit
- Diagnostic reagent kit
- Rapid lateral flow test
- Immunoassay rapid diagnostic test
- Molecular test kit
- Cassette rapid diagnostic test
- Rapid virus test
- Respiratory infection test kit
- Whole blood detection kit
- Serum rapid diagnostic test
- Plasma rapid diagnostic test
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.