- Laboratory
- Laboratory medicine
- Rapid infectious disease test
- Shenzhen unimed-global technology Co.,LTD

- Products
- Catalogs
- News & Trends
- Exhibitions
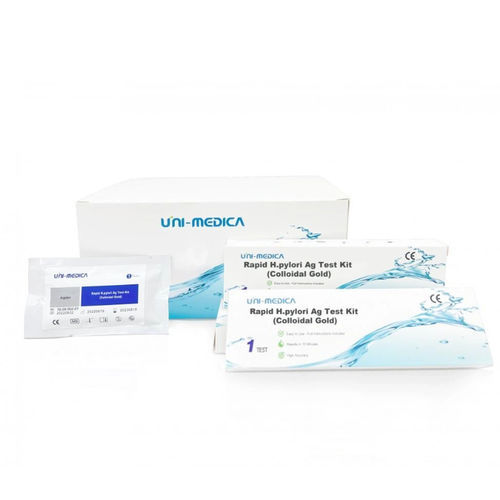
Rapid infectious disease test 3822009020for antigensHelicobacter pyloriclinical
Add to favorites
Compare this product
Characteristics
- Applications
- for infectious diseases
- Tested parameter
- for antigens
- Micro-organism
- Helicobacter pylori
- Sample type
- clinical, feces, fecal, laboratory
- Analysis mode
- lateral flow
- Format
- cassette, strip
Description
in feces specimens to aid in the diagnosis of H. pylori infection.
The test is intended for use by trained clinical laboratory personnel and individuals trained in point of care settings.
For professional use only. For in vitro diagnostic use only.
Principle of The Test
The Rapid H.pylori Ag Test Kit employs a lateral flow chromatographic technology to qualitatively detect the H. pylori antigens in fecal specimens. The fecal sample is collected and treated with the extraction buffer. Add the processed specimen into the Test Cassette, the specimen will migrate forward along with the test strips through capillary effect. If the H.pylori is present in the specimen, the H.pylori antigens will be captured and detected on the T line, resulting in purplish red band on the test region, indicating a positive result. If the H.pylori is not present or present at very low levels in the specimen, there is no red line appears in T line position. The “Control Line” (C) is used for procedural control. Control line should always appear if the test procedure is performed properly and the test reagents are working.
Storage and Stability
1. The test device is sensitive to humidity and as well as to heat;
2. Store kit components at 2-30°C, out of direct sunlight;
3. Kit components are stable until the expiration date printed on the pouch or kit box;
4. Use the Test cassette immediately after removing the test device from a foil pouch, the
Test Cassette must be used within 1 hour or discarded.
5. Do not freeze.
Other Shenzhen unimed-global technology Co.,LTD products
Colloidal Gold Platform
Related Searches
- Assay kit
- Solution reagent kit
- Blood assay kit
- Molecular biology reagent kit
- Serum assay kit
- Immunoassay assay kit
- Plasma assay kit
- Infectious disease detection kit
- Blood rapid diagnostic test
- Protein reagent kit
- Diagnostic reagent kit
- Rapid lateral flow test
- Immunoassay rapid diagnostic test
- Molecular test kit
- Cassette rapid diagnostic test
- Rapid virus test
- Respiratory infection test kit
- Whole blood detection kit
- Serum rapid diagnostic test
- Plasma rapid diagnostic test
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.