- Laboratory
- Laboratory medicine
- Infectious disease test kit
- Sichuan Xincheng Biological Co., LTD
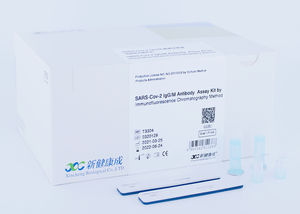
Genetic assay kit for neutralizing antibodyvirusSARS-COV-2
Add to favorites
Compare this product
Characteristics
- Tested parameter
- genetic, for neutralizing antibody
- Micro-organism
- virus, SARS-COV-2, coronavirus
- Sample type
- cell, blood, biological
- Analysis mode
- immunochromatographic, immunofluorescence
- Result display time
Min.: 8 min
Max.: 15 min
- Sample volume
0.005 ml, 0.0085 ml
(0.00017 US fl oz, 0.00029 US fl oz)
Description
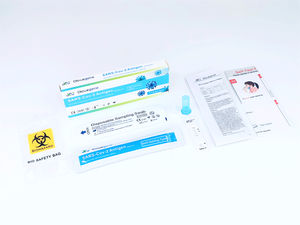
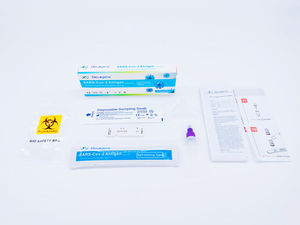
The Diacegene SARS-CoV-2 Neutralizing Antibody Assay Kit (Immunofluorescence) is used as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection, it should not be used to diagnose acute SARS-CoV-2 infection.
About SARS-CoV-2 Neutralizing Antibodies
Infection by the SARS-CoV-2 or immunized by vaccine against SARS-CoV-2 will induce immune responses, such as the production of antibodies in the blood. These antibodies can specifically bind with virus receptor binding domain (RBD) to block the binding of RBD with ACE2 on human cells surface, thus prevent the virus or its genetic material into the human cell and virus infection. These antibodies are named neutralizing antibodies.
The SARS-CoV-2 neutralizing antibodies are protective antibody produced by the human body after inoculation with novel coronavirus vaccine or infection with novel coronavirus. The kit is used to monitor the presence of neutralizing antibodies in subjects vaccinated with the novel coronavirus vaccine or in people infected with the novel coronavirus, it can be used to evaluate the immune effect after vaccination or whether neutralizing antibodies are produced in human body after infection with novel coronavirus, it should not be used to diagnose acute SARS-CoV-2 infection.
Applicable Instrument:
Immunofluorescent Analyzer (IFP-3000), Immunofluorescent Analyzer (IFP-2000) produced by Sichuan Xincheng Biological Co., Ltd.
Catalogs
No catalogs are available for this product.
See all of Sichuan Xincheng Biological Co., LTD‘s catalogsOther Sichuan Xincheng Biological Co., LTD products
COVID-19
Related Searches
- Assay kit
- Solution reagent kit
- Blood assay kit
- Serum assay kit
- Immunoassay assay kit
- Plasma assay kit
- Infectious disease detection kit
- Respiratory infection test kit
- Whole blood detection kit
- Clinical assay kit
- Clinical chemistry reagent
- Lateral flow test kit
- COVID-19 detection kit
- Clinical chemistry analyzer
- Antigen assay kit
- IgG test kit
- Automatic clinical chemistry analyzer
- Strip detection kit
- Benchtop clinical chemistry analyzer
- Laboratory detection kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.