- Laboratory
- Laboratory medicine
- COVID-19 test kit
- Sichuan Xincheng Biological Co., LTD
COVID-19 assay kit IgMIgGcoronavirus
Add to favorites
Compare this product
Characteristics
- Applications
- COVID-19
- Tested parameter
- IgM, IgG
- Micro-organism
- coronavirus
- Sample type
- serum, plasma, whole blood, nasal, biological, throat
- Analysis mode
- immunofluorescence
- Format
- strip
- Result display time
8 min
- Sample volume
0.005 ml, 0.0085 ml
(0.00017 US fl oz, 0.00029 US fl oz)
Description
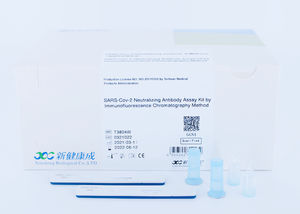
Novel Coronavirus (2019-nCoV) IgM/IgG antibody Fast Test Kit (Immunofluorescence Assay) is intended for the qualitative detection of 2019-Novel Coronavirus IgM and IgG antibody in serum, plasma or whole blood samples from patients suspected of COVID-19 infection by a healthcare provider.
About 2019-nCoV
The novel coronaviruses belong to theβgenus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases.
Applicable Instrument:
Immunofluorescent Analyzer (IFP-3000), Immunofluorescent Analyzer (IFP-2000) produced by Sichuan Xincheng Biological Co., Ltd.
Immunofluorescent Analyzer (AFS-1000) produced by Guangzhou Labsim Biotech Co., Ltd.
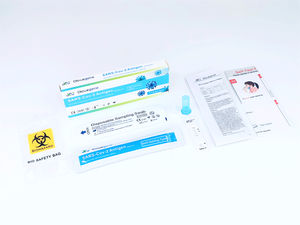
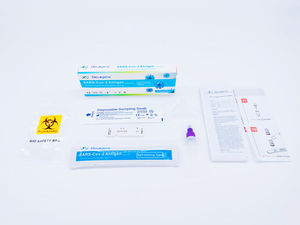
Contents:
1) Test card: It consists of reagent strip and plastic bag.
2) ID chip card
3) Sample diluent
4) Capillary tube
5) User manual
Catalogs
No catalogs are available for this product.
See all of Sichuan Xincheng Biological Co., LTD‘s catalogsOther Sichuan Xincheng Biological Co., LTD products
COVID-19
Related Searches
- Assay kit
- Solution reagent kit
- Blood assay kit
- Serum assay kit
- Immunoassay assay kit
- Plasma assay kit
- Infectious disease detection kit
- Respiratory infection test kit
- Whole blood detection kit
- Clinical assay kit
- Clinical chemistry reagent
- Lateral flow test kit
- COVID-19 detection kit
- Clinical chemistry analyzer
- Antigen assay kit
- IgG test kit
- Automatic clinical chemistry analyzer
- Strip detection kit
- Benchtop clinical chemistry analyzer
- Laboratory detection kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.