
- Company
- Products
- Catalogs
- News & Trends
- Exhibitions
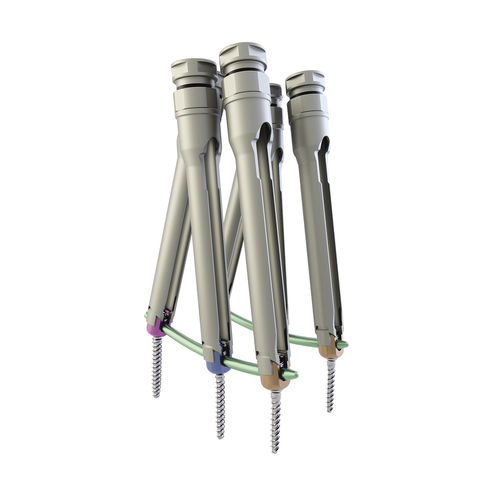
Thoraco-lumbar osteosynthesis unit mont blanc MISposterioradult
Add to favorites
Compare this product
Characteristics
- Spinal section
- thoraco-lumbar
- Surgical approach
- posterior
- Patient type
- adult
Description
The mont blanc MIS implants consist of various forms and sizes of screws, locking screws and rods.
The mont blanc MIS implants can be combined for creating a posterior spinal implant system in a minimally invasive approach and/or open approach.
They are used with dedicated instruments (depending on the surgical approach) which allow preparation of the site, insertion of implants and removal.
The mont blanc MIS implants are supplied for single use.
Implants material: titanium alloy (ISO 5832-3).
Instruments material: stainless steel, silicone, polyphenyl sulfone, POM, nitinol, ABS and polypropylene.
The mont blanc MIS system is intended to provide immobilization and stabilization of spinal segments in skeletally mature patients as an adjunct to fusion in the treatment of acute and chronic instabilities or deformities of the thoracic, lumbar and sacral spine.
This mont blanc MIS system is intended for posterior noncervical pedicle fixation and non-pedicle fixation (from T1 to S1) utilizing a percutaneous minimally invasive approach for the following indications in skeletally mature patients:
degenerative disc disease (defined as back pain of discogenic origin with degeneration of the disc confirmed by history and radiographic studies);
spondylolisthesis;
trauma (i.e., fracture or dislocation);
spinal stenosis;
deformities (i.e., scoliosis, kyphosis, and/or lordosis);
spinal tumor,
pseudoarthrosis; and
failed previous fusion.
The mont blanc MIS system is intended to be used in a posterior minimally invasive approach and/or open approach.
The mont blanc MIS system is intended to be used with autograft and/or allograft.
Exhibitions
Meet this supplier at the following exhibition(s):

Related Searches
- Interbody fusion cage
- PEEK interbody fusion cage
- Lumbar interbody fusion cage
- Anterior interbody fusion cage
- Screw-rod unit
- Posterior spinal fusion system
- Cervical interbody fusion cage
- Adult spinal fusion system
- Posterior interbody fusion cage
- Transforaminal interbody fusion cage
- Thoraco-lumbar spinal fusion system
- Thoraco-lumbar interbody fusion cage
- Pedicle screw positioning system
- Oblique interbody fusion cage
- Pediatric spinal fusion system
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.






