- Furniture, Logistics
- Hospital ward, Hosting
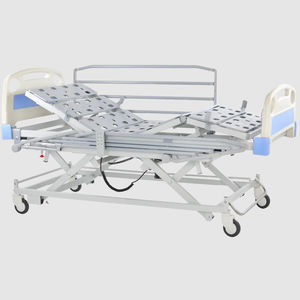
- Hospital bed
- Tecnimoem Care S.L
Hospital bed TEIDE LOWelectricheight-adjustableTrendelenburg
Add to favorites
Compare this product
Characteristics
- Applications
- hospital
- Operation
- electric
- Features
- height-adjustable, Trendelenburg, reverse Trendelenburg, ultra-low
- Component
- on casters, with locking wheels
- Options
- double
- Number of sections
- 4-section
- Width
190 cm
(74.8 in)- Length
90 cm, 105 cm
(35.4 in, 41.3 in)- Weight capacity
150 kg
(330.7 lb)
Description
The product is intended to be used in humans for the purpose of treating or alleviating a disease, as well as for the treatment, alleviation or compensation of an injury or a deficiency, according to the current Spanish regulations on medical devices. According to the UNE-EN 60601-2-52 standard for medical beds, it can be used for two different application environments (EA):
•EA3.- long-term care provided in a medical area where medical supervision is required and monitoring is provided if necessary and the bed is used in medical procedures that can be provided to help maintain or improve the patient's condition.
•EA4.- care provided in a domestic area where the bed is used to alleviate or compensate an injury, disability or illness.
The bed is considered a class 1 medical device according to the European Regulation 2017/745 on Medical Devices (MDR). Reference rules:
* UNE-EN 60601-2-52 “Electrical medical equipment. Particular requirements for the basic safety and essential function of medical beds.
MATTRESS BASE External frame in 50x20x1.5mm steel tube and internal joints in 25x25x1.5mm steel tube curved at corners (manufactured according to UNE 10305-5). Safety distance of 30 mm all around the bed between the joints and the outer frame to avoid entrapment. Beige epoxy-polyester paint (consult other options). Provided with squares in the head area for anchoring drip support and/or incorporator. Possibility of mounting metallic or wooden side safety railings. Metal mattress holder on the footboard of the inner articulation and injected thermoplastic mattress holder (PP with fiberglass) on the sides.
Catalogs
No catalogs are available for this product.
See all of Tecnimoem Care S.L‘s catalogsRelated Searches
- Tecnimoem care hospital bed
- Tecnimoem care tilting bed
- Trendelenburg bed
- Hospital bed mattress
- Tecnimoem care medical bed
- Reverse Trendelenburg bed
- Foam mattress
- Fixed-height bed
- Bedside cabinet with compartments
- Shower and bath seating with backrest
- Healthcare facility armchair
- Hygiene chair shower and bath seating
- Shower and bath seating with armrests
- Bedside cabinet with drawers
- Over-bed table on casters
- Tecnimoem care nursing home bed
- Height-adjustable over-bed table
- Mobile shower and bath seating
- Shower and bath seating with cutout seat
- Waterproof mattress
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.