- Dental
- Dental practice
- Xenograft bone substitute
- Tecnoss Dental

- Products
- Catalogs
- News & Trends
- Exhibitions
Xenograft bone substitute GTO®for dental sugerygranules

Add to favorites
Compare this product
Characteristics
- Type of graft
- xenograft
- Use
- for dental sugery
- Shape
- granules
Description
Tissue origin :
Heterologous cortico-cancellous bone mix
Tissue collagen :
Preserved
Physical form :
Pre-hydrated granules and TSV Gel
Composition :
80% granulated mix, 20% TSV Gel
Granulometry :
600-1000 µm
Re-entry time :
About 5 months
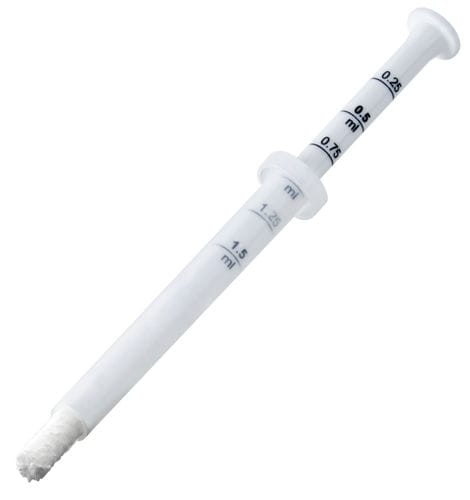
Packaging :
Syringe: 0.5 cc
Wide tip syringe: 2.0 cc
Characteristics
Heterologous bone grafting material made of a mix of collagenated cortico-cancellous granules of size ranging from 600 to1000 µm, properly blended with TSV Gel, which is a mixture of heterologous type I and III collagen gel with polyunsaturated fatty acids and a biocompatible synthetic copolymer diluted in aqueous solution. GTO® is gradually resorbed and it is extremely osteoconductive. Moreover, the preserved collagen matrix characterizing the granules facilitates blood clotting and the subsequent invasion of repairing and regenerative cells. These unique properties guarantee an excellent rate of new bone formation, delivering adequate graft volume preservation, a healthy new bony tissue and, ultimately, a successful implant rehabilitation. The presence of the same kind of granules of its progenitor, mp3®, which are very similar to human mineral bone, assures a similar biological response of the host tissue. GTO® can be used as alternative to autologous bone.
Handling
GTO® is available in two sizes (0.5 and 2.0 cc) as ready-to-use pre-hydrated biomaterial and can be easily grafted to the defect site. Thus, clinicians can skip the hydration step with saline or blood, saving time and decreasing the risk of accidental exposure to pathogens.
VIDEO
Exhibitions
Meet this supplier at the following exhibition(s):

*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.






