- Laboratory
- Laboratory medicine
- COVID-19 test kit
- Tianjin Guyufan Biological Technology

- Products
- Catalogs
- News & Trends
- Exhibitions
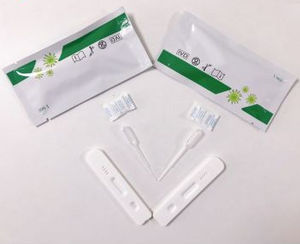
COVID-19 test kit for home usefor antigensSARS-COV-2
Add to favorites
Compare this product
Characteristics
- Applications
- COVID-19
- Application field
- for home use
- Tested parameter
- for antigens
- Micro-organism
- SARS-COV-2, coronavirus
- Sample type
- blood, nasopharyngeal, saliva
- Analysis mode
- for PCR
- Other characteristics
- self-test
Description
CategoriesTesting and vaccines
Brand gyf
Model COVID-19-wantai-hesuan
Certification FDA
Certification CE
Certification CFDA
specification 48pcs/box
Unit Price US $ 2-5 / piece
FOB port All ports in the country
Terms of Payment L/C, D/A, D/P, Western Union
The "new coronavirus (2019-nCoV) IgM antibody detection kit (immunochromatography)" has the following advantages:
(1) Rapidly screen for new coronary pneumonia within 15 minutes, shorten the waiting time of patients, provide timely treatment, and reduce the workload of designated hospitals for the epidemic.
(2) The sample source is flexible, with whole blood, serum and plasma, the operation is very simple.
(3) Single test, without any equipment, no restrictions on operators and operating space, suitable for on-site screening
Other Tianjin Guyufan Biological Technology products
Testing And Vaccines
Related Searches
- Assay kit
- Blood assay kit
- Serum assay kit
- Immunoassay assay kit
- Plasma assay kit
- Rapid lateral flow test
- Immunoassay rapid diagnostic test
- Molecular test kit
- Cassette rapid diagnostic test
- Rapid virus test
- Respiratory infection test kit
- Infectious disease rapid diagnostic test
- COVID-19 detection kit
- Rapid respiratory infection test
- ELISA assay kit
- Antigen assay kit
- IgG test kit
- COVID-19 rapid diagnostic test
- Nasopharyngeal assay kit
- Antibody assay kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.