- Laboratory
- Laboratory medicine
- COVID-19 rapid test
- ulti med Products (Deutschland)
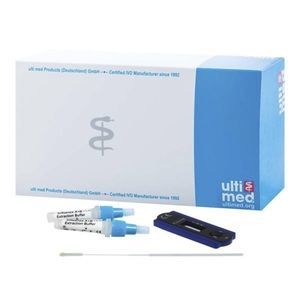
COVID-19 rapid test 012G521for antigensproteincoronavirus
Add to favorites
Compare this product
Characteristics
- Applications
- COVID-19
- Tested parameter
- for antigens, protein
- Micro-organism
- coronavirus
- Sample type
- saliva
- Analysis mode
- immunoassay, lateral flow
- Format
- strip
- Result display time
10 min
Description
The ulti med COVID-19 Antigen Saliva Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from 2019-nCoV in saliva specimens directly collected from individuals who are suspected of COVID-19 by their healthcare provider within the first 7 days of symptom onset. Results are for the identification of 2019-nCoV nucleocapsid protein antigen.
PRINCIPLE
The ulti med COVID-19 Antigen Saliva Test uses double-antibody sandwich to detect the antigen of novel coronavirus (2019-nCoV) in saliva samples. During detection, the gold labeled anti-2019-nCoV monoclonal antibody in the labeling pad binds to the 2019-nCoV antigen in the sample to form a complex, and the reaction complex moves forward along the nitrocellulose membrane under the action of chromatography. It is captured by the anti-2019-nCoV monoclonal antibody pre-coated on the detection zone (T) on the nitrocellulose membrane, and finally a red color reaction line is formed in the T zone. If the sample does not contain 2019-nCoV antigen, a red color reaction line cannot be formed in the T zone. If the sample does not contain 2019-nCoV antigens, a red reaction line cannot be formed in the T zone. Regardless of whether the sample to be tested contains 2019-nCoV antigens, a red reaction line will always form in the quality control area (C).
Advantages
Painless sampling due to saliva collection in the mouth
Higher acceptance by test subjects
Higher willingness for prophylactic, frequent testing
Simplest test performance without handling, such as diluting and pipetting, with a possibly infectious sample
Catalogs
No catalogs are available for this product.
See all of ulti med Products (Deutschland) ‘s catalogsOther ulti med Products (Deutschland) products
Covid-19, Infl. A/B & RSV Tests
Related Searches
- Assay kit
- Blood rapid diagnostic test
- Rapid lateral flow test
- Immunoassay rapid diagnostic test
- Cassette rapid diagnostic test
- Rapid virus test
- Serum rapid diagnostic test
- Plasma rapid diagnostic test
- Clinical assay kit
- Infectious disease rapid diagnostic test
- Whole blood rapid diagnostic test
- Lateral flow test kit
- Rapid respiratory infection test
- Urine rapid diagnostic test
- Strip detection kit
- Test strip
- COVID-19 rapid diagnostic test
- Bacteria rapid diagnostic test
- Strip rapid diagnostic test
- Urine assay kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.