- Laboratory
- Laboratory medicine
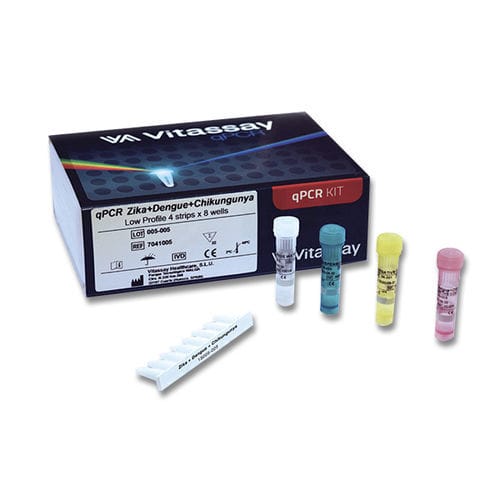
- Dengue fever test kit
- VITASSAY HEALTHCARE S.L.
Dengue fever test kit 7041005Zika viruschikungunyavirus
Add to favorites
Compare this product
Characteristics
- Applications
- dengue fever, Zika virus, chikungunya
- Micro-organism
- virus
- Sample type
- clinical, laboratory
- Analysis mode
- for RT-PCR
Description
Vitassay qPCR Zika+Dengue+Chikungunya test allows the detection and differentiation of Zika, Dengue and/or Chikungunya viruses by real-time RT-PCR in clinical samples. The product is intended for use in the diagnosis of Zika virus, Dengue virus and/or Chikungunya virus infections alongside patient clinical data and other laboratory tests outcomes.
Transport and storage
• The resuspended positive control should be stored at -20ºC. In order to avoid
repeated freeze/thaw cycles, it is recommended to distribute the content in
different aliquots.
• Keep all reagents in the darkness.
Additional equipment and material required
• RNA extraction kit
• Real-time PCR instrument (thermocycler) (Attached I)
• Centrifuge for 1.5 mL tubes
• Vortexer
Micropipettes (1-20 µL, 20-200 µL)
• Filter tips
• Powder-free disposal gloves
Summary
Zika (ZIKV), Dengue (DENV) and Chikungunya (CHIKV) viruses are transmitted by the
sting of the same Aedes genus mosquito.
Besides, Zika virus was isolated in 1947 from a rhesus monkey in the Zika forest in
Uganda. Typically, clinical manifestations of human ZIKV disease are similar to many
others derived of arbovirus infections that occur without serious complications and may
include a self-limiting febrile illness, arthralgia, myalgia, headache and maculopapular
rash. Conjunctivitis, retroorbital eye pain, lymphadenopathy and diarrhoea have also
been reported.
Catalogs
Vitassay_Catalogue
20 Pages
Related Searches
- Assay kit
- Solution reagent kit
- Blood assay kit
- Molecular biology reagent kit
- Plasma assay kit
- Infectious disease detection kit
- Blood rapid diagnostic test
- Diagnostic reagent kit
- Rapid lateral flow test
- Enzyme reagent kit
- Immunoassay rapid diagnostic test
- Molecular test kit
- Cassette rapid diagnostic test
- Rapid virus test
- Respiratory infection test kit
- Optical assay kit
- Clinical assay kit
- Infectious disease rapid diagnostic test
- Buffer solution reagent kit
- Fluorescence assay kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.