Traumatology software reportingviewersharing
Add to favorites
Compare this product
Characteristics
- Function
- reporting, viewer, sharing, for communication
- Applications
- medical, surgical, for traumatology
- Type
- real-time
- Deployment mode
- for smartphones, for tablet PC
Description
A comprehensive software solution tailored for trauma care providers to expedite patient care and improve outcomes
Review imaging and communicate with the entire care team––anytime, anywhere
Streamlined care. Exceeding new standards.
Review images and case context
Respond and connect with the entire care team
Team activation at the touch of a button
Team activation and alerts
Activate your entire care team to expedite decision-making and treatments
Alerts appropriate surgical specialists
Retains timestamps for guideline reporting and performance improvement initiatives
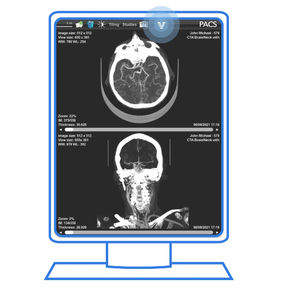
High-fidelity image viewer
Access from your phone
Display various imaging modalities
Improve image sharing across referring and treating centers
Workflow and communication
Streamline workflow
Simple access to imaging and critical information–anytime, anywhere
Communicate with the entire team with the push of a button
Meeting ACS trauma standards every time.
Level 1 and 2 trauma centers require continuous availability of human and physical resources to initiate endovascular or interventional radiology procedures for hemorrhage control within 60 minutes of request.
IR acceptance to arterial access With 60 mins
Ortho response to patient bedside With 30 mins
Neurosurg response time and evaluation With 30 mins
Catalogs
No catalogs are available for this product.
See all of Viz.ai‘s catalogs*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.