Arm mobility rehabilitation system ALEX RSlimbs mobilityshoulder mobilityrobotic
Add to favorites
Compare this product
Characteristics
- Therapy type
- arm mobility, limbs mobility, shoulder mobility
- Technology
- robotic, virtual
- Options
- with serious games, with interactive workout, with gamification applications, with virtual reality system
Description
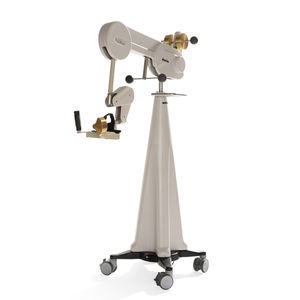
ALEx RS is founded on an innovative design utilizing a novel tendon actuated transmission to provide patients with exceptional comfort, lightweight, and transparency during use. It is a CE certified medical device classified as IIa. The design of ALEx RS originates from the extensive tradition in robotic design established at the Scuola Superiore Sant’Anna and its application in clinical rehabilitation post-stroke.
Features
ALEx is a six Degrees of Freedom (DoFs) mechanically compliant exoskeleton designed for the upper human limb. The exoskeleton includes four sensorized and actuated joints for shoulder abduction-adduction (SH-Abd-Ad), rotation (SH-Rot), and flexion-extension (SH-Flx-Ext), and elbow flexion-extension (EL-Flx-Ext). Additionally, it features two sensorized and passive joints for forearm pronation-supination (FO-Pro-Sup) and wrist flexion-extension (WR-Flx-Ext).
Thanks to its unique shoulder joint kinematics, the exoskeleton is easy to wear and can be adjusted to various anthropometric sizes effortlessly. ALEx can cover approximately 92% of the natural arm workspace, enabling robotic assistance during the execution of natural spatial movements.
Benefits
Robotic devices offer intensive and high frequency rehabilitation treatment, accompanied by continuous performance monitoring and movement biomarkers to customize treatment levels based on the patient’s requirements.
Exoskeleton systems enable precise control over joint movements and articulation torques.
ALEx RS can offer guided assistance during intricate upper limb motions, adjusting assistance levels automatically or manually to suit the patient’s needs.
Catalogs
Brochure ALEx RS
8 Pages
Related Searches
- Rehabilitation system
- Robotic rehabilitation system
- Rehabilitation system with serious games
- Arm mobility rehabilitation system
- Rehabilitation system with interactive workout
- Limbs mobility rehabilitation system
- Virtual rehabilitation system
- Rehabilitation system with gamification applications
- Shoulder mobility rehabilitation system
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.