- Laboratory
- Physico-chemical analysis
- Liquid chromatography system
- Zhejiang JYSS Bio-Engineering Co., Ltd.

- Company
- Products
- Catalogs
- News & Trends
- Exhibitions
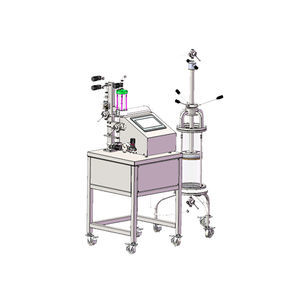
Liquid chromatography system AutoPrepfor biopharmaceutical applicationspilotfor protein purification
Add to favorites
Compare this product
Characteristics
- Type
- liquid
- Applications
- for biopharmaceutical applications, pilot, for protein purification
- Other characteristics
- high-throughput, compact
Description
AutoPrep Automated Chromatography System is applicable to the pilot scale and large-scale production of bio-pharmaceutical purification processes such as monoclonal antibody, insulin, blood products, vaccine and recombinant protein. As an integrated control unit, it can automatically realize the column equilibration, sample loading, elution, collection, regeneration and cleaning in the purification and separation process with compliance of GMP guideline. The system can provide a variety of flexible configurations to meet the needs of large-scale production.
Features and Advantages
Compact design, stack designed pipeline, and minimal dead volume
Comply with USP class VI material and provide complete validation documents
Comply with FDA 21CFR Part 11, Electronic Records Electronic Signatures : real time recorded data and logs, with traceable and unmodifiable.
Flexible and powerful method editor, can realize automatic process production based on time, volume and column volume.
Catalogs
No catalogs are available for this product.
See all of Zhejiang JYSS Bio-Engineering Co., Ltd.‘s catalogsOther Zhejiang JYSS Bio-Engineering Co., Ltd. products
Purification and Filtration
Related Searches
- Chromatograph
- Chromatography column
- LC chromatography system
- Compact chromatography system
- Chromatography pump
- Chromatography system for the pharmaceutical industry
- High-throughput chromatography system
- High-capacity chromatography column
- Quaternary chromatography pump
- Chromatography column for pharmaceutical applications
- High-throughput chromatography column
- Pilot chromatography system
- Proteomic chromatography system
- Protein purification chromatography column
- Protein purification chromatography system
- Chromatography system for biopharmaceutical applications
- Protein separation chromatography column
- Scientific research chromatography column
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.