- Laboratory
- Laboratory medicine
- COVID-19 rapid test
- Zhejiang Orient Gene

- Company
- Products
- Catalogs
- News & Trends
- Exhibitions
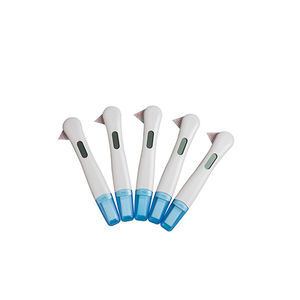
COVID-19 rapid test professionalfor antigensSARS-COV-2nasopharyngeal
Add to favorites
Compare this product
fo_shop_gate_exact_title
Characteristics
- Applications
- COVID-19
- Tested parameter
- for antigens
- Micro-organism
- SARS-COV-2
- Sample type
- nasopharyngeal, nasal
- Analysis mode
- immunochromatographic
- Format
- cassette
- Result display time
15 min
- Sample volume
0.08 ml
(0.00271 US fl oz)- Specificity
97.5 %
- Sensitivity
100 %
Description
The COVID-19 IgG/IgM (Whole Blood/Serum/Plasma) Rapid Test Device utilizes lateral flow technology for the qualitative, differential detection of both anti-SARS-CoV-2 IgM and IgG antibodies. In general, antibodies can be detected 1-3 weeks after infection. This test is intended to screen patients for SARS-CoV-2 antibodies.
Coronaviruses are enveloped RNA viruses that are distributed broadly among humans, other mammals and birds that cause respiratory, enteric, hepatic and neurologic diseases. Four viruses - 229E, OC43, NL63 and HKU1 are prevalent and typically cause common cold symptoms in immunocompromised individuals. Three other strains SARS-CoV, MERS-CoV and SARS-CoV-2 (COVID-19) can be transmitted from between non-human vertebrates to humans.
Detection Window (IgM): 3-5 days after incubation
Dual band results for simple interpretation
Multivariable analysis of immunoglobin IgG & IgM
Room temperature storage or refrigerated (2-30⁰C / 36-86⁰F)
Procedural internal control included
Buffer included
Clinical Evaluation
Positive Percent Agreement (PPA): IgG 96.7%; IgM 86.7%; Overall 96.7%
Negative Percent Agreement (NPA): IgG 98.0%; IgM 99.0%; Overall 97.0%
Clinical Agreement with Characterized Samples
Sensitivity: IgG 96.7%; IgM 100%; Combined 100%
Specificity: IgG 97.5%; IgM 100%; Combined 97.5%
Specimen: Whole Blood, Serum, Plasma
Shelf Life: 24 months from the date of manufacture
Catalogs
No catalogs are available for this product.
See all of Zhejiang Orient Gene‘s catalogsRelated Searches
- Assay kit
- Blood assay kit
- Molecular biology reagent kit
- Serum assay kit
- Immunoassay assay kit
- Plasma assay kit
- Infectious disease detection kit
- Blood rapid diagnostic test
- Rapid lateral flow test
- Immunoassay rapid diagnostic test
- Molecular test kit
- Cassette rapid diagnostic test
- Virus rapid diagnostic test
- Respiratory infection test kit
- Whole blood detection kit
- Serum rapid diagnostic test
- Plasma rapid diagnostic test
- Optical assay kit
- Clinical assay kit
- Infectious disease rapid diagnostic test
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.